As mentioned in the previous article of the series, it is customary to refer to stochastic or random effects at low radiation doses. This means that statements about the probability of sickness are only possible for a group of people and not for individuals. However, radiation biology cannot assess whether and where such mutations will be caused. Thus, it remains unclear whether sickness will occur in individual cases, and if so, which kind of sickness.
A robust risk assessment is a useful way of raising awareness of a prudent radiation protection approach. Therefore, determining this risk is one of the central tasks of epidemiology in combination with radiation biology.
How dose values affect the risk of sickness
The International Commission on Radiological Protection ICRP has introduced a model that illustrates the relationship between the dose and the risk derived from it: the LNT model, also called the Linear No-Threshold Model. According to the LNT model, the linear relationship is considered to apply for acute doses starting from approximately 100 millisievert (mSv).
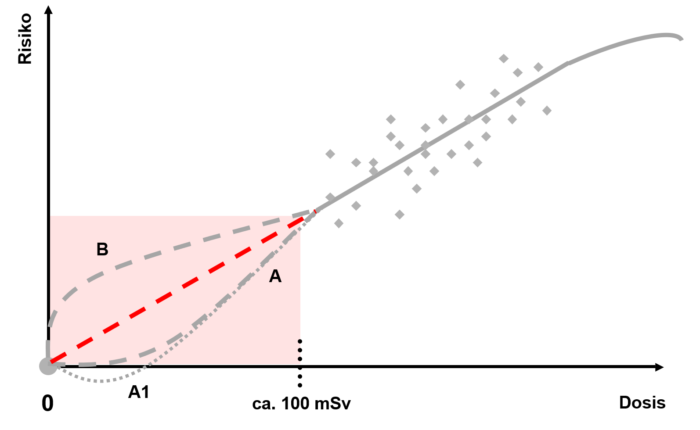
Illustration: ENSI
However, for doses of less than 100 mSv it is not clear whether they have a linear effect on the risk of sickness. In this respect, there have been various epidemiological studies that have come to different conclusions. Some report a statistically relevant increased risk of cancer between 0 and 100 mSv, while other studies do not find any correlation. However, the international expert committees UNSCEAR and ICRP subject each of these studies to a critical review and formulate recommendations that ENSI fundamentally follows.
According to the LNT assumption (red line in this graph), each low dose results in a proportional increase in risk. However, this very lowdose range is associated with a very high degree of uncertainty, because, under certain circumstances, the dose-induced risk is smaller than the risk that already exists spontaneously. As a result, it is almost impossible to distinguish the dose-induced risk from the spontaneous risk. In this range, risk predictions are only possible based on statistical calculations and estimates. Therefore, the UNSCEAR and ICRP committees have warned against use of the LNT model to calculate the risk of very low dose values. It is also not suitable for calculating the number of expected cancer cases.
As a cautious and therefore conservative assumption, the LNT model in Switzerland is taken into account in the fully revised Radiological Protection Ordinance (in force since 2018). ENSI primarily uses the LNT model to assess optimisation measures in operational radiation protection.
How the body handles low doses of radiation
From the results of radiobiological experiments it can be concluded that cells can handle very low doses very well thanks to their repair system. This would lead to a theoretical risk that is less than the expected risk (curve A in the graph). The theory of the biopositive effect was derived from very low doses (curve A1). A very low dose could cause the cells’ repair system to be activated, thus protecting them from future damage, but in itself not causing any noticeable negative effects.
The cells’ molecular repair system has developed so that it can be used throughout the cell nucleus. Ionisations that can lead to mutations occur, even at low doses, along a relatively concentrated “radiation path”. Here, the repair molecules must first reach the possible damage locations and then be positioned there. During this time, the effects of radiation cannot be eliminated or can only be partially eliminated (curve B). Therefore, at least initially, the resulting risk would be greater than expected.
Since neither of the two theories has been empirically confirmed, a cautious assumption in radiation biology is the compromise that there is a linear correlation between dose and the resultant risk – even at low doses.
Ionising radiation as a trigger for cell mutations
It has been scientifically recognised for some time now that ionising radiation can contribute to the development of cancer. Less well known were the genetic mechanisms that cause cell mutations. This has changed in recent years and decades.
Illustration: iStock.com/selvanegra
Through the discovery and investigation of the so-called oncogenes (cancer genes), scientists have found basic processes with which normal cells become cancer cells along various paths. Comparing non-irradiated and irradiated cells shows which effects are triggered by ionising radiation.
In Switzerland, several tens of thousands of people spontaneously develop cancer every year. Advances in medicine in the areas of early detection and therapy have significantly increased the chances of recovery from many types of cancer.
This is the third of five articles on radiation biology. The fourth part considers the measurement of ionising radiation.
More information
Cover image: iStock.com/Svisio